SARS-CoV-2 has left thousands with a distorted or lost sense of smell. This population is helping researchers identify what happens when smell goes wrong and what might help
It’s been over 2 years since the pandemic began, and COVID-19 still dominates small talk. Sharing personal experiences with the disease was also how the conversation started when Christine Kelly, who lives in London, spoke with C&EN: “I got it three times,” she says. “The last one I had was Delta. And it completely wiped out my sense of smell.”
It wasn’t the first time Kelly’s sense of smell had disappeared. She had lost it in 2012 after a different viral infection, and the loss had a devastating effect on her. It took 2 years after that infection for Kelly to regain some quality of life, she says. “I had been in a flat, gray, featureless desert of sensory input and also experienced profound depression during that 2-year period,” she says. Even years later, as the SARS-CoV-2 virus spread around the world, she was still noticing new smells returning, so “when I got my first case of COVID and it put a dent in that recovered sense of smell, the anxiety was greater than the actual issue,” she says.
“When I got to the third case, that completely wiped out my sense of smell. I was just in a terrible, terrible state. Terrible,” she recalls.
Perhaps it’s surprising that losing smell would cause such a large emotional response. But we are surrounded by a world of chemical information that we perceive through our sense of smell, often without even noticing. Of all the human senses, our sense of smell is the oldest evolutionarily, according to Pamela Dalton of the Monell Chemical Senses Center. In our brains, our emotional system came later and was built on top of the olfactory system. The physical intertwining may explain why smell can evoke strong memories and emotions.
COVID-19’s olfactory effects aren’t confined to a loss of smell. For some, infection causes the smells that people typically relish or find comfort in—like the morning’s coffee— to trigger a full-throttle sense of disgust or revulsion.
Kelly is well placed to explain the overwhelming effects that a loss and distortion of smell can wreak and how the pandemic brought those changes into focus. In May 2019, she set up AbScent, a charity to provide information and support to those who had experienced olfactory loss. The act was prescient because less than a year later, her small organization was flooded with thousands of new people looking for help.
Before 2020, around 12% of the US population over age 40 had an impaired sense of smell, according to the US National Institutes of Health. And then came the pandemic. “We live in a changed world when it comes to olfactory problems,” Kelly says. “We used to worry about, ‘How do we raise awareness?’ Well, those days are gone. We have awareness, and now it’s a whole new game.”
The sudden huge number of people affected by postviral smell disorders has also allowed researchers to better understand how viruses can alter the sense of smell long after the virus has left the body.
“Postviral [olfactory dysfunction] pre-COVID was really not well understood because we never had enough people with the same virus at the same time,” says Dalton, who studies how people perceive odor. “Now we have potentially millions of people who will experience these changes, and we’ll be able to more definitively link loss of olfaction or changes in olfaction to long-term health.”
Those whose smell has been affected by COVID-19 have already helped researchers propose new mechanisms for smell loss, and these findings indicate new ways to help improve life for those with disrupted smell.
A DELICATE SENSE
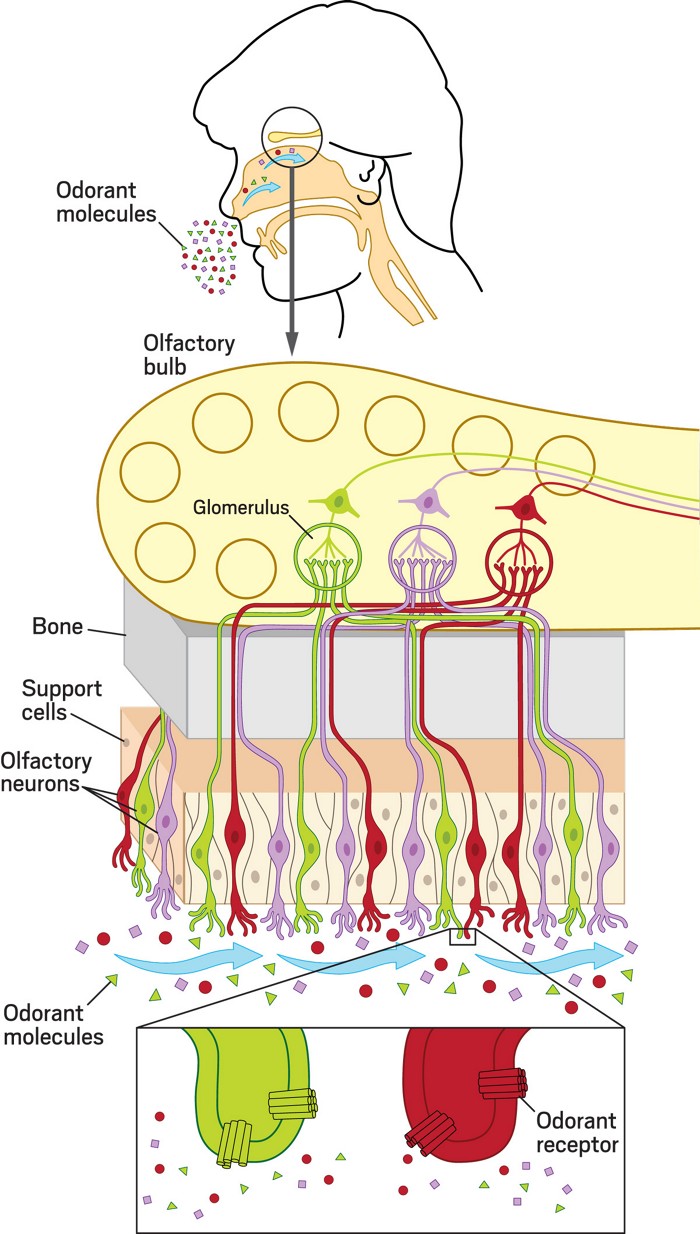
After odor molecules in the air float into the nose, they settle on the nasal cavity surface. From there, they diffuse through a mucous layer before latching on to the binding pockets of olfactory receptor proteins sitting in olfactory neurons’ membranes. Unlike neurons involved in other senses, the neurons that detect odorant molecules are in direct contact with the outside world. That position makes them uniquely exposed and very sensitive.
Dalton explains that we may have the capacity to distinguish thousands of smells or even more. But the nose has only around 400 different odor receptors. And each neuron inside the nose has only one type of receptor. That means that each receptor has to be able to bind multiple scent molecules. Each combination of molecules creates a particular pattern of signals from the receptors. When our brain recognizes the pattern, we can identify the smell.
A molecule’s binding to an odor receptor changes the receptor protein’s 3D structure. That change starts a chemical cascade inside the nerve cell that results in a signal at the other end, where the cell connects to the olfactory bulb. The connections inside the bulb help sort those signals. Neurons with the same type of receptor send their signals to a common hub, called a glomerulus. The brain decodes the pattern of activated glomeruli to recognize a particular smell, perhaps of our partner or pet.
“It’s pretty amazing,” Dalton says, pondering that slight differences from person to person could mean that we all experience smell slightly differently.
Multiple things, including head trauma, nasal polyps, neurodegenerative diseases, and viral infections, can reduce people’s ability to smell. But researchers hadn’t known all the ways that viral infections could cause lasting smell damage. That is, until the beginning of 2020, when the pandemic hit.
MORE THAN JUST A STUFFY NOSE
With many respiratory viruses, a loss of smell is pretty easy to understand. The nose becomes inflamed and full of mucus. Simply put, the odor molecules can’t make it to the smell receptors in a stuffy nose. When the symptoms go away, the smell loss usually does too.
Many people with COVID-19 who lose their sense of smell do so quickly. Dalton describes it as having a switch turned off. And while for most, the sense of smell returns to normal in a week or two, COVID-19-associated smell loss can last for months.

Once researchers realized COVID-19 was affecting smell, several turned their attention to the question of how it did so—and particularly whether the olfactory nerves themselves got infected with the virus. If so, infected nerves could have let the virus into the brain. But studies of postmortem tissue from the noses of people who were infected with SARS-CoV-2 ruled that idea out: scientists confirmed that the neurons in the nose don’t have the type of receptor (called the angiotensin-converting enzyme 2, or ACE2, receptor) that SARS-CoV-2, uses to infect cells.
But in the nose, the neurons that detect odor molecules are surrounded by what are called support cells, which hold the neurons in place. It’s the support cells that can get infected, damaged, and die, says Thomas Hummel, an olfaction expert at the Interdisciplinary Center for Smell and Taste. Although the virus directly attacks the support cells, this can indirectly damage the olfactory neurons.
During the acute phase of infection, the support cells can pump out molecules that activate an inflammatory response to try to fight the virus. These inflammatory factors can get into the olfactory neurons and change them, says Marianna Zazhytska, a postdoc at Columbia University.
Until 2020, Zazhytska had been studying smell loss related to Alzheimer’s disease. As part of that work, she and her colleagues had shown that the folding of the genes inside olfactory neurons is different from that in most other cells in the body. In olfactory neurons, the DNA is arranged like a fried egg: the center of the nucleus is filled with tightly wound DNA, while key genes for smell, like those for olfactory receptors, are spread out in loops away from the center. In people with Alzheimer’s, this arrangement gets scrambled.
Early reports of smell loss in people with COVID-19 quickly caught Zazhytska’s attention, and she and her adviser, Stavros Lomvardas, pivoted their research direction as the SARS-CoV-2 virus tore through New York.
By analyzing human postmortem samples from people infected with SARS-CoV-2 as well as hamster models of the disease, Zazhytska found that during infection, inflammatory factors from the support cells affect DNA organization in the neurons in the same way Alzheimer’s does. This reorganization causes many olfactory neurons’ proteins to not be made. In the short term, the lack of proteins stops the neurons from detecting smells during infection. But in some people, the organizational changes might not reverse, causing longer-lasting smell loss (Cell 2022, DOI: 10.1016/j.cell.2022.01.024).
The gene scrambling might also be one reason for the disordered sense of smell—a phenomenon known as parosmia—that some experience as smells start to return.
SCENTS OF DISGUST
Since mid-2020, internet searches and social media posts using the word parosmia have rocketed. In late June 2020, the Facebook group that AbScent’s Kelly set up for people with post-COVID-19 smell loss became overwhelmed with posts about the topic.
Parosmia, she explains, is often incredibly distressing, and while there are many triggers, the biggest problems center on feelings of disgust when around certain foods. Sometimes these foods can’t be avoided. “Just imagine,” she says, “going to the grocery store, handling the food, putting it in the shopping cart, paying for it, bagging it, unbagging it, putting it away, preparing it, cooking it, God forbid bringing it to the table, and then maybe feeding children. Watching disgusting food disappear into the mouths of other people. I mean, it’s awful.”
THE OLFACTORY SYSTEM
In 2019, Kelly gave a talk at a conference about her experiences of parosmia. In the audience was Jane K. Parker, a chemist and founder of the Flavour Centre at the University of Reading and now a board member of AbScent. It was the first time Parker had heard of the phenomenon, she recalls. “I was there as a flavor chemist, and I’d heard of anosmia, but parosmia was completely from left field. Where did that come from?”
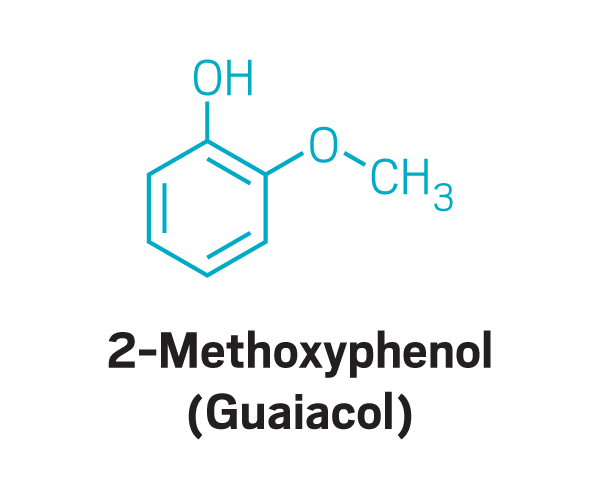
As she listened to Kelly describe the foods, such as meat and coffee, that were her parosmia triggers, Parker recognized the foods as all containing potent aroma compounds produced by a specific set of reactions. “It’s all Maillard chemistry,” she says. Maillard reactions are the ones responsible for the browning of foods during cooking or roasting. Sugars and amino acids react to produce fragrant compounds that include chemicals such as pyrazines and thiols.
To explore the phenomenon, Parker worked with 30 people with postviral parosmia. Half the cases were from COVID-19, and half from other viruses. Each person sat in front of a gas chromatograph that separated a substance’s aroma compounds one by one, allowing participants to smell individual compounds. In parallel, the researchers identified the compounds by mass spectrometry. This setup allowed the researchers to determine individual odor compounds that were causing the problems.
GAG ORDERS
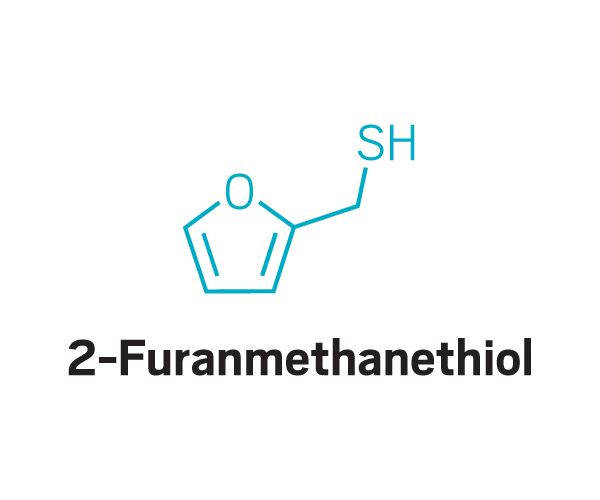
These odor compounds, found in substances like coffee and meat, triggered parosmia—a distorted sense of smell—in people who took part in a study led by Jane Parker at the University of Reading.
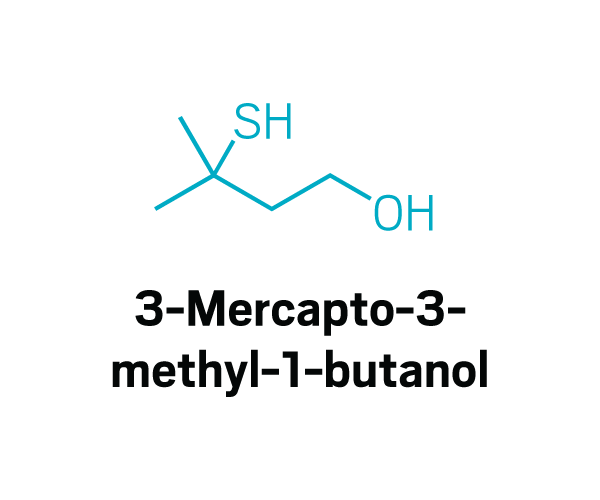
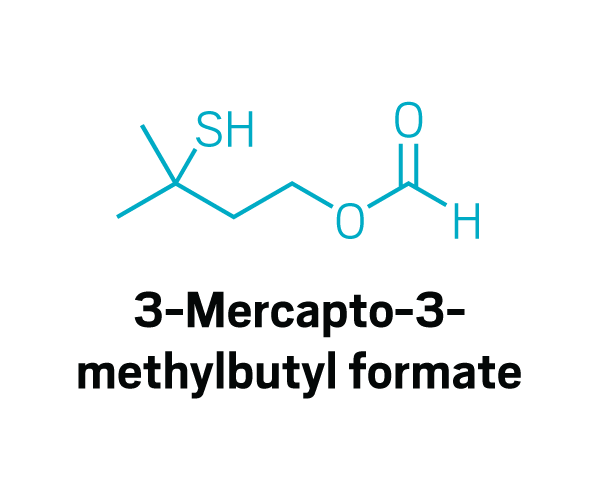
Thiols
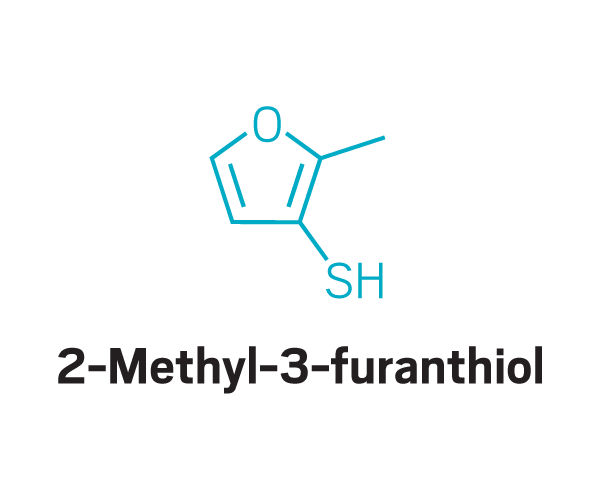
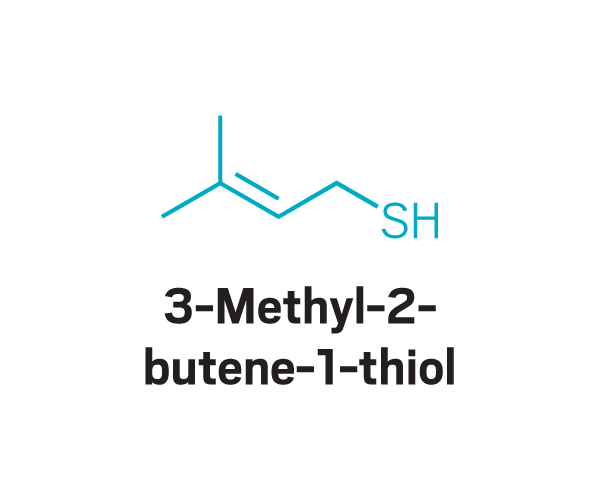
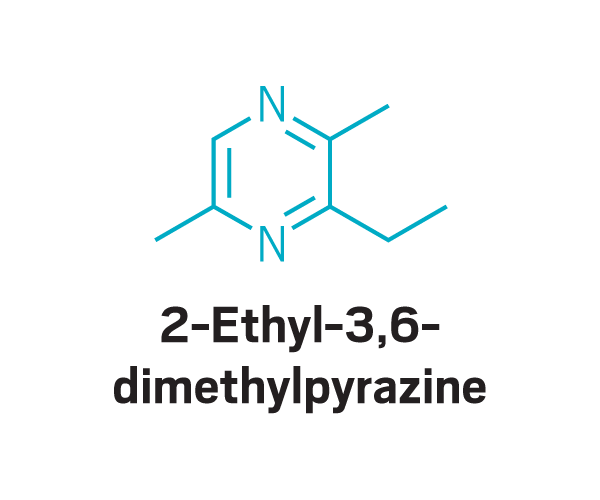
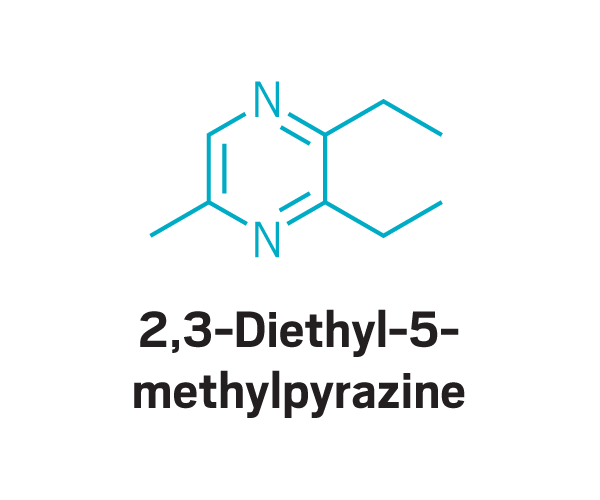
Trisubstituted pyrazines
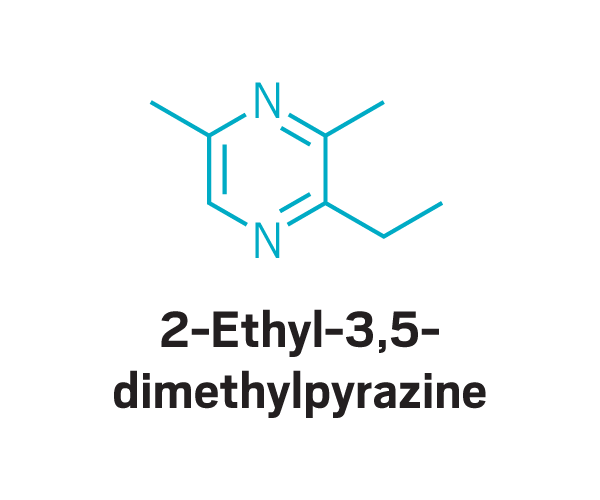
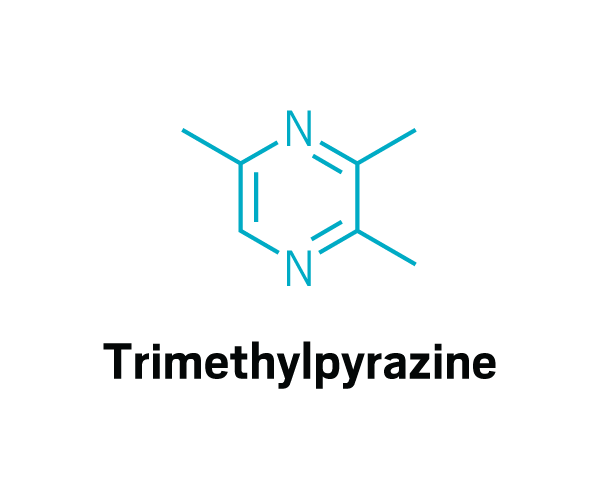
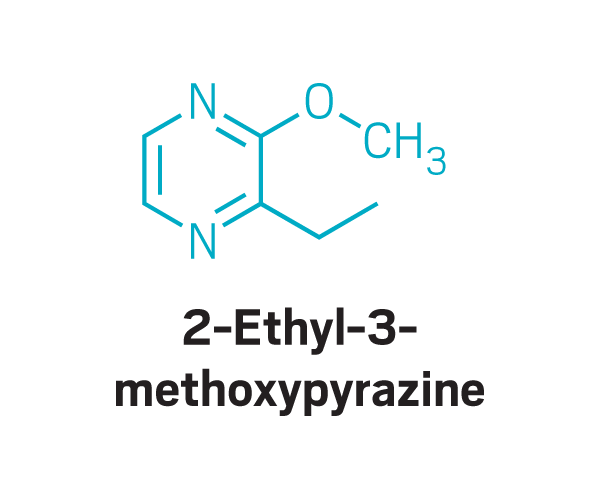
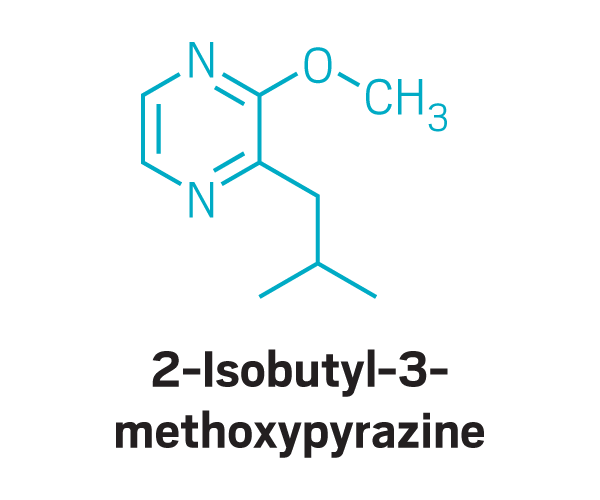
Methoxy pyrazines
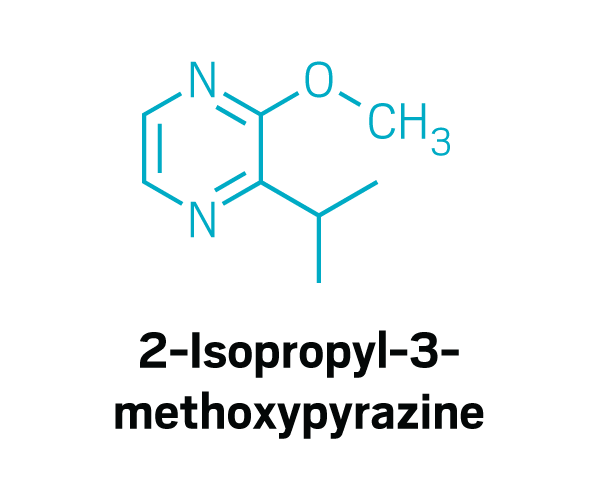
Disulfides
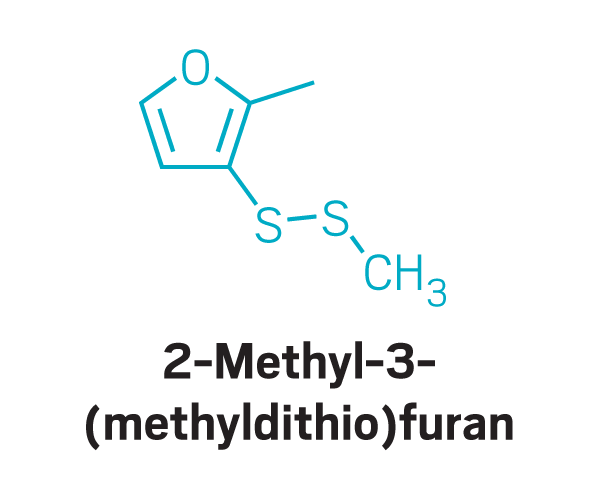
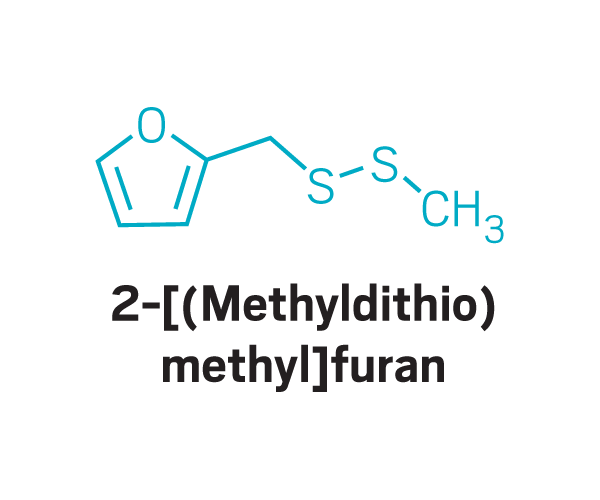
Other
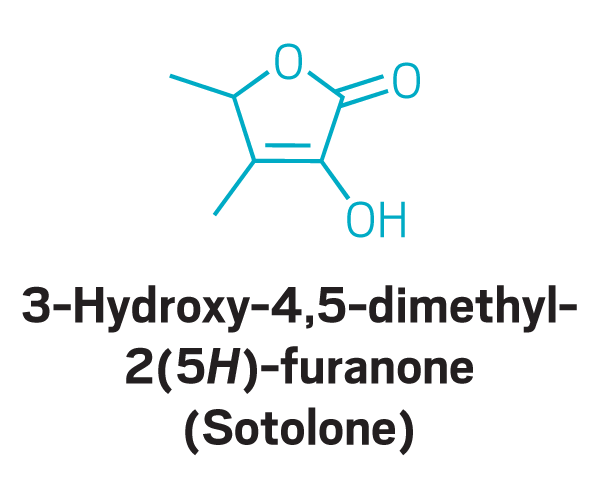
“The first day, it was just a light bulb moment,” Parker recalls. The first participant sat in front of Parker’s machine, sniffed the compounds that were being released, and immediately identified a trigger molecule as it came out of the instrument. “The first person said, ‘That’s parosmia,’ ” Parker says. “She could recognize that compound as being one of the ones that was causing her sense of disgust. And then 3 min later, she got another one.”
Participants identified as disgusting the same compounds again and again. These included 2-furanmethanethiol and 2-ethyl-3,6-dimethylpyrazine, most commonly found in coffee, and 2,3-diethyl-5-methylpyrazine, found in roasted, fried, and baked goods, grilled chicken, and peanut butter (Commun. Med. 2022, DOI: 10.1038/s43856-022-00112-9). In total, around 15 compounds tended to trigger disgust in the participants who worked with Parker.
“Most people enjoy coming through the lab and find it hugely beneficial just to talk about it,” Parker says. Many are also grateful to realize that they aren’t making things up. “So many people think it’s in their head,” she says. “So to come along to a lab session and find out that it’s triggered by this molecule . . . that really helped people come to terms with the fact that it was real.”
Zazhytska, who discovered that COVID-19 could alter gene organization in olfactory neurons, speculates that disorganized DNA expression might be involved in parosmia—for example, by causing a different receptor gene to be expressed in a particular neuron. All of a sudden, a neuron isn’t picking up the scent molecules that it’s wired for, so it sends a signal to a different glomerulus than the scent would normally trigger. Such mixed-up connections are an appealing explanation to Parker too.
While participants of these studies have helped researchers find possible explanations for lost or altered sense of smell, how can this information help improve the conditions of the people affected?
SNIFFING OUT CURES
A recent meta-analysis led by Song Tar Toh at Singapore General Hospital estimated that while most people regain their sense of smell and taste after COVID-19, around 5% of people with COVID-19 might experience persistent changes in their sense of taste or smell (BMJ 2022, DOI: 10.1136/bmj-2021-069503). Although that study followed people for only about 6 months, Kelly believes the effects may last much longer. She estimates that the number of people affected will run into the millions worldwide.
Smell and taste loss are invisible disabilities, says Duncan Boak, who lost his sense of smell in 2005 after a head injury. Like Kelly, who set up AbScent to provide materials and support for people like her, Boak has also established a patient-focused charity, called Fifth Sense, to transform how smell and taste disorders are recognized, treated, and studied. Since 2020, he says, the charity’s work has ramped up significantly. One of its priorities for research is to determine how different treatments might help rehabilitate a lost or disordered sense of smell, Boak says.
Understanding the mechanisms of olfaction and olfactory cell regulation and the reasons that smell loss occurs will help drug discovery efforts, says medicinal chemist Graham Wynne, who has recently joined Fifth Sense’s advisory board. “COVID hasn’t done many good things. But it’s really shone the spotlight on the condition [of smell loss] and the impact that it has on the people that it affects, and that stimulates research, of course.” He sees parallels with other sensory disabilities. For example, some drugs are now in early to midstage human trials that take regenerative approaches to treating hearing loss. Perhaps there are analogous ways to fix damaged sense of smell.
Some treatments for regenerating the sense of smell are already in clinical trials. For example, a team of researchers at Stanford University is testing whether injections of platelet-rich plasma could help the renewal of olfactory cells. Other researchers are running trials to see if topical vitamin A, otherwise known as retinol, could help regenerate olfactory neurons. Retinoic acid, a metabolite of vitamin A, has important roles in tissue development and regeneration.
Researchers are also looking at transplanting tissues. For example, Bradley Goldstein of Duke University restored the sense of smell in mice using cultured stem cells grafted into the nose (Stem Cell Rep. 2019, DOI: 10.1016/j.stemcr.2019.05.001).
Still others are building olfactory implants that stimulate the olfactory system with electrodes, somewhat like cochlear implants. Hummel of the Interdisciplinary Center for Smell and Taste is part of a European consortium to develop these implants. “This is very exciting,” he says. “But this will only be tailored for a limited number of patients and for a certain patient group.” Those with more extreme olfactory loss, for example, are more likely to be willing to undergo the required surgery.
One approach Hummel developed has already been shown to help: smell training. In smell training, individuals sniff a sequence of four essential oils, inhaling each one for 15 s while concentrating on their memory of the corresponding smell. They repeat the procedure twice a day over the course of months.
Hummel used rose, eucalyptus, lemon, and clove in his first studies because he expected their odor compounds to activate a broad range of receptors and because these odors are strong and well recognized. But the choice of scents isn’t as important as the consistent use, according to Hummel.
Kelly says following this approach helped get her smell back. “Against all odds, I’m recovering. And I’m well on my way to being back with my sense of smell to where it was before the pandemic,” she says. This includes being able to perceive ambient smells, she says, something that usually comes late in recovery.
Smell training is akin to meditation, she says. “It should really be called smell awareness. I am aware of smell 24 h a day. And that, I think, is key to training,” she says.
The changes induced by smell training aren’t just subjective but can be measured—for example, using clinical tests for odor detection and discrimination, Hummel says. Recent research by Aytug Altundag of the Biruni University School of Medicine has shown that smell training can help people with parosmia recover more quickly than those who don’t do smell training (Laryngoscope 2022, DOI: 10.1002/lary.30101). And people don’t have to wait to get their sense of smell back before starting smell training, according to both Parker and Hummel. Often, some neurons might still be working without a person’s being able to perceive it, so this training should start to help rebuild the connections between those neurons and the processing centers in the brain. That rebuilding is one of the main ideas behind smell training, Hummel says: the extra stimulation “helps neurons to regenerate or regenerate faster than they would do normally.”
DISTILLING THE ESSENCE
Researchers continue to find ways to improve a damaged sense of smell, which means people shouldn’t give up hope. “In previous postviral olfactory disorders, it’s not unusual for people to have gone 2 years and then suddenly start to get their sense of smell back,” Dalton says, so the same may happen for those who had COVID-19.
That improvement matters to those affected, Boak says. The knock-on impacts for many people are multifaceted and incredibly isolating, he says. For example, not being able to smell a partner or enjoy food can lead to people feeling alone and removed from important relationships. That can stop people from going out, or can make them feel vulnerable. Depression and anxiety can often result. And there are also safety issues. Boak says he nearly blew himself up after returning to his flat and not smelling that the stove had been leaking gas into the kitchen for hours. Fifth Sense has now partnered with a UK gas distribution network to raise awareness among the public of the crucial role that smell plays in personal safety. They hope to be able to educate people who have lost their sense of smell about how to keep themselves safe—for example by using natural gas detectors.
“There has never been this level of interest in the field in the past,” Boak says. But he cautions to not forget the people behind the data. “We need people to understand the myriad impacts of not being able to smell,” he urges.













