The sight of grandchildren’s faces, the smell of home cooking, the sound of a friend’s voice: We process so much of life through our eyes, ears, and nose. While many older adults experience some decline in their senses as they age, there is growing scientific evidence that these changes may also be related to dementia and overall brain health.
About one third of older adults have some form of vision problems or loss by age 65, and nearly 50% of people older than 75 have disabling hearing loss. Anosmia, which is the medical term for the decline or loss of smell, can also be a significant blow to quality of life. Still, for most older adults, these common signs of aging don’t affect cognitive health.
Too many older adults consider sensory decline to be something that they must learn to live with, yet there are treatment options available. Scientists are studying whether the risk of cognitive problems can be reduced when these conditions are treated — with glasses, eye surgery, hearing aids, or other health care approaches. Read on to learn how NIA-funded investigators are finding new insights into how the eyes, ears, and nose might be windows into our overall cognitive health.
Eye-brain connections
Alison Abraham, Ph.D., M.S., M.H.S., associate professor of epidemiology and ophthalmology at the University of Colorado School of Public Health, has long studied the relationship between the eyes and the brain. She is a principal investigator with the NIA-funded Eye Determinants of Cognition (EyeDOC) Study.
Abraham has a family history of vision problems that has fueled her personal interest in how vision loss affects emotional well-being, physical functioning, social interaction, and brain health. In her work, she collaborates with community groups and care providers on strategies for increasing routine vison screening and broadening access to vision care for older adults.
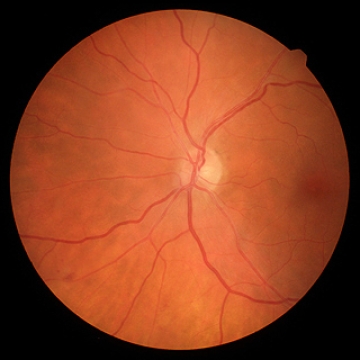
Abraham’s research focuses on the retina, the area at the rear of the eyeball that relays visual information through the optic nerve to the brain. “In the womb, the development of the retina and the brain are closely linked,” said Abraham.
In the EyeDOC study, Abraham uses a noninvasive imaging method called optical coherence tomography (OCT) to examine the retina. OCT is currently used to diagnose and manage diseases, including diabetes-related retinopathy and glaucoma. Compared to more intrusive brain imaging tests such as MRIs or PET scans, OCT provides an easier, less expensive way to learn about the health, thickness, and architecture of retinal nerves and blood vessels. Initial studies with OCT have shown obvious differences in retinal nerve fiber thickness between cognitively healthy older adults and people with Alzheimer’s disease and related dementias.
Abraham is continuing studies in this area and is hopeful that OCT could be repurposed to develop retinal biomarkers for earlier screening of people at risk for cognitive impairment. Other researchers found that cataract extraction was significantly associated with a lower risk of dementia development.
A recipe for retina-based scans
Like Abraham, Maya Koronyo-Hamaoui, Ph.D., M.S., a professor in the Cedars-Sinai Department of Neurosurgery, is also studying the retina’s close connections to the brain for clues about cognitive health and the risk of brain disease.
In recent years, Koronyo-Hamaoui and her colleagues have discovered the first evidence of the specific diagnostic signs of Alzheimer’s disease-related amyloid beta protein plaques in retinas from Alzheimer’s and mild cognitive impairment patients. In a series of several NIA-funded innovative studies, her team found increased retinal amyloid plaques and associated retina blood vessel and nerve cell degeneration in patients compared to age and sex-matched cognitively healthy individuals.
Her team also created a low-cost, noninvasive technique to detect Alzheimer’s protein plaques at a very high resolution in the retinas of living patients. Additionally, they developed a modified version of a powerful eye imaging tool — the scanning laser ophthalmoscope — that includes a wider look at previously overlooked peripheral regions in the retina. They combine this scan with giving research participants an oral dose of curcumin, a natural compound that lends zest and orange color to Indian spices such as curry and turmeric. Curcumin is naturally drawn to amyloid beta, the protein that comprises Alzheimer’s disease plaques in the brain, making it easier for the researchers to spot.
“You can look at the retina and see things at the molecular, cellular, and vascular levels, like protein aggregates and vascular abnormalities,” said Koronyo-Hamaoui. Her team is exploring if optical retinal imaging with curcumin could be a cost-effective test to identify Alzheimer’s pathology, including in people with mild cognitive impairment. “We uncovered parallels between the effects of Alzheimer’s disease in the retina and brain, and that specific changes in the retinal regions mimic changes in the brain and cognitive status,” she added.
Koronyo-Hamaoui’s team is collaborating with neuropathology experts across the United States, Europe, and Australia to expand their studies into Alzheimer’s retinopathy and visual deficits to see if a similar test can detect buildup of the protein tau, another hallmark of the disease, in the retina. Their hope is to develop affordable, accessible retina-based scanning technology that might someday be used in combination with routine cognitive and brain imaging for the earlier detection of Alzheimer’s and related dementias, Parkinson’s disease, and stroke.
Vision loss can snowball social isolation
Untreated vision loss can be especially devastating. Using data from the Health and Retirement Study, NIA-funded scientists recently made the case for including vision impairment as a risk factor for dementia, estimating that nearly 100,000 dementia cases in the U.S. could possibly have been prevented with existing vision treatments.
Older adults stop driving, they stop going out, they have difficulty reading, and they don’t exercise as much because they’re afraid of falling. Untreated vision problems really reduce social interaction, emotional well-being, and physical activity, which are all risk factors for cognitive decline.
Hearing loss and dementia
The eyes aren’t the only sensory organs connected to memory and cognition. Research has also linked hearing with brain health. Two experts at the Johns Hopkins Bloomberg School of Public Health and School of Medicine — Jennifer Deal, Ph.D., an epidemiologist and gerontologist, and Frank Lin, M.D., Ph.D., director of the Cochlear Center for Hearing and Public Health — are exploring whether untreated hearing loss contributes to cognitive decline in older adults. They are also working to find medical and policy solutions to the problem.
Lin’s interest in the field began as a child when he witnessed the impact of his grandmother’s hearing loss on her quality of life. Deal began her career in basic science but grew interested in studying dementia and cognitive decline in hopes of making a real difference for older adults. While in training to become an ear, nose, and throat surgeon, Lin came across a JAMA paper published nearly two decades earlier, in 1989, that showed a clear association between greater hearing loss and the chances of having dementia.
“It really struck me because it made sense to me clinically based on what I observed in my patients,” Lin said. “But through mid-2009, as I was finishing my ENT training, little research had been done to build on those findings and explore the connection further.” Around this time, Lin met NIA Scientific Director Luigi Ferrucci, M.D., Ph.D., who encouraged him to dive in to the rich Baltimore Longitudinal Study of Aging (BLSA) data on the topic.
In 2011, Ferrucci, Lin, and colleagues published a landmark paper on the connections between dementia and hearing. Research since then has repeatedly confirmed these connections, with the 2020 Lancet Commission on Dementia ranking hearing loss as the single largest potentially modifiable risk factor for dementia.
Overloading memory circuits
What exactly is the biological connection between hearing problems and cognition? “In someone who has hearing loss, they end up with a garbled signal that gets sent to the brain, making it more difficult to decode,” Deal said. Untreated hearing loss forces the brain to work harder, which ultimately overwhelms the compensating brain networks normally used for memory and thinking. This effect is known as cognitive load, or effortful listening.
Older adults with untreated hearing loss often seek to avoid the embarrassment of struggling to hear friends and family, or the difficulty of understanding conversations during errands, shopping, or other daily routines. This can lead to social isolation resulting in less sensory stimulation, which compounds the problem as the sound processing areas of the brain can begin to shrink.
Since their 2011 paper, Lin, Deal, and colleagues have continued to investigate if treating hearing loss can help stave off this destructive cycle and slow down cognitive decline. To find out, they are soon finishing up a five-year clinical trial known as the Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study. ACHIEVE is tracking nearly 1,000 older adults with hearing loss across the United States to compare thinking and memory skills between those who have their hearing loss treated (for example, with hearing aids) and control groups who are not actively treating their hearing loss.
Protect your hearing and get your hearing loss treated
The take-home advice for older adults is to protect your hearing from loud noises that can damage it. The delicate cells in our inner ear that encode sound do not regenerate over time like skin, heart, or liver cells. “What you’re born with is what you get,” Lin said.
Lin, Deal, and colleagues lead a public awareness campaign, hearingnumber.org, that explains how adults can now measure their own hearing at home with a smartphone, similar to how individuals may self-monitor their own blood pressure, weight, or glucose. The statistics show that most older adults will eventually have some meaningful hearing impairment, so it’s important to self-monitor your own hearing regularly or get it checked by a professional. If you are diagnosed with hearing loss, get it treated.
-
Increasing access to hearing treatment
The cost of hearing aids is a major barrier to treating hearing loss. Lin notes that less than 20% of Americans with hearing loss actually get hearing aids. “Hearing aids are incredibly expensive, and Medicare does not cover hearing aids or any related hearing care services,” he said. Lin has long worked with government, scientific, and medical groups to push for increased access to affordable hearing aids. In August 2022, in response to previous bipartisan Congressional legislation passed in 2017 and at the urging of the White House, the Food and Drug Administration released new rules to expand access to lower cost, over-the-counter (OTC) hearing aids that became available to consumers in late 2022. To learn more about OTC hearing aids, visit www.nidcd.nih.gov/health/over-counter-hearing-aids.
Smell as a gateway to memory
The sense of smell is closely linked with memory, possibly more so than any of our other senses, and declining smell sensitivity can foreshadow dementia. A team of NIA scientists found that for BLSA participants, poorer scores on smell sensitivity tests predicted mild cognitive impairment and had connections to dementia-related pathology seen in brain scans.
Mark Albers, M.D., Ph.D., a neurologist at Massachusetts General Hospital, has found further evidence that change in the sense of smell can serve as an early warning sign for neurodegenerative diseases. Additionally, Albers is working with a startup company to develop an at-home scent test that can be scored online.
Albers and his teammates study the olfactory bulb region — rounded masses of brain tissue above each nasal cavity that house nerve cells involved in smell. This region of the brain is also part of a circuit of neurons involved in memory. “Because of that direct wiring, there’s an intimate connection,” Albers said. “For some reason, that circuit is particularly vulnerable to types of pathologies that give rise to Alzheimer’s disease. Our hypothesis is that change in a person’s ability to smell is the ‘canary in the coal mine,’ where we’re able to detect those changes even before people develop memory symptoms.”
That hypothesis, which was first tested in mouse models, has also been supported in human studies. Albers led a study of smell sensitivity in a cohort of more than 200 human study participants whose cognitive ability ranged from normal cognition through mild cognitive impairment and into dementia. Volunteers were given a multiple-choice smell test to gauge how well they could detect, identify, and differentiate scents. The researchers found that loss of smell sensitivity correlated with biomarkers of Alzheimer’s, including the thinning of tissue in the regions of the brain associated with memory.
“Before people had symptoms, we found that decline in smell sensitivity was a warning signal that showed that their memory was starting to fail,” Albers said. “We’ve been able to demonstrate that many of those patients went on to develop mild cognitive impairment.” Albers and his team are using specialized algorithms to help fine-tune the smell sensitivity tests.
Albers encourages older adults who are experiencing declines in their sense of smell to see their doctors to rule out other causes. He notes that smell loss in neurodegenerative diseases such as Parkinson’s disease or Lewy body dementia is usually profound enough that people notice it right away, but Alzheimer’s disease-related smell decline is very gradual.
Sight and sound: A key combination for cognition and independence
Dual declines in vision and hearing are especially dangerous for brain health. A study led by scientists at the University of Washington School of Public Health showed that compared with adults who have no sensory impairment, those with “dual” hearing and vision loss are at higher risk for developing some form of dementia.
“There’s a big movement now to recognize that sensory loss doesn’t happen in isolation, that oftentimes people may have more than one deficit in sensory function,” said Deal.
Shannon L. Risacher, Ph.D., associate professor at the Indiana University School of Medicine, is another scientist studying the intersections of multiple senses and dementia risk. Rather than focusing on only one sensory domain, she has recently branched out to explore the effect of decline in multiple sensory systems on cognition, as well as their link to amyloid beta and tau in the brain. “There have been five or six study publications in just the last few years that have suggested that this sort of change across multiple senses is a particularly big flag for whether or not somebody is at risk for Alzheimer’s disease,” Risacher said.
She pointed out that other studies have shown that people with parallel declines in hearing, vision, and smell have a higher risk for progression to Alzheimer’s compared with those who have impairment in only one sense. Her team is currently exploring how social isolation and lack of stimulation caused by multiple sensory loss can accelerate cognitive decline.
When you start to have multiple sensory changes, that puts you at almost double the dementia risk compared to a single sensory change. So, if you start to have changes in your vision and hearing at the same time, and particularly if you have a family history of Alzheimer’s disease or another form of dementia, go and get it checked out by your doctor.
Today, working with the Alzheimer’s Disease Research Center at Indiana University, Risacher is among several investigators across the country with a growing interest in the impact of multiple senses on cognition. Scientists from the University of California, San Francisco, recently tracked more than 1,800 people ages 70 to 79 for a decade who initially had normal cognitive health. They found strong connections between lower combined sensory test scores and higher risk for dementia over that time period, even for those with mild sensory loss.
While the two most common sensory problems can be daunting, they are also very treatable. “I think that we tend to feel like losing your hearing and vision is just a part of aging,” said Abraham. “Many older adults don’t take them as seriously as they do diabetes or cardiovascular disease. But hearing and vision loss are two big contributors that are highly correctable. Get your vision checked out, get reading glasses from your local drugstore, get your hearing checked. It’s important for your quality of life.”
Excitement for the future
While it’s essential to work with your doctor to address potentially treatable conditions, such as hearing or vision loss, or declines in smell sensitivity, there is excitement on the horizon for new tests and techniques to improve cognitive health as we age. Stay connected with NIA to learn more about the latest developments, including more affordable diagnostic tests and new tools for earlier detection and treatment of sensory and cognitive problems.
Source: Exploring sensory decline and dementia risk | National Institute on Aging