Study: Non-invasive Imaging of Sense of Smell by Tracking the Voltage-Gated Sodium Channel NaV1.7. Image Credit: Axel_Kock / Shutterstock.com
However, this condition can also be caused by genetic disorders, traumatic injuries, and mutations in the ion channels that allow messages to be transmitted from the olfactory neurons to the brain. Most commonly, mutations in the voltage-gated sodium channel 1.7 (Nav1.7) have been known to lead to either total or partial loss of smell.
In a recent study published on the preprint server bioRxiv*, researchers discuss a new method of tracking the Nav1.7 channel to reduce the reliance on more invasive diagnostic measures.
An overview of Nav1.7
Nav1.7 is important for both perceiving pain and olfactory sensing. Olfactory disorders can cause significant distress for affected individuals, as they can alter taste, as well as reduce appetites, and cause difficulties with cooking, social interactions, and maintaining personal hygiene.
Nav1.7 is highly expressed in more peripheral sensory neurons and the axons of human olfactory sensory neurons (OSNs). This sodium channel is responsible for relaying olfactory information to the higher-order neurons in the brain.
Nav1.7 is encoded for by the SCN9a gene. Even small mutations in this gene are known to cause a loss of function of the olfactory epithelium/bulb (ROEB). These injuries can be caused by a viral infection; however, this is not the cause of coronavirus disease 2019 (COVID-19)-related loss of smell.
About the study
In order to detect which cases of anosmia are caused by the loss of function of Nav1.7, the researchers created a fluorescently labeled molecular probe (Tsp1a-IR800) specific to Nav1.7 that can act as a marker for olfaction. The probe showed clear success, with the olfactory epithelium/bulb visible using fluorescence.
Furthermore, images obtained by the probe showed high contrast between areas that expressed Nav1.1, such as the ROEB and surrounding regions. The results were far clearer and showed a massive increase in radiant efficiency as compared to mice injected with phosphate-buffered saline (PBS) or treated with a blocking formulation.
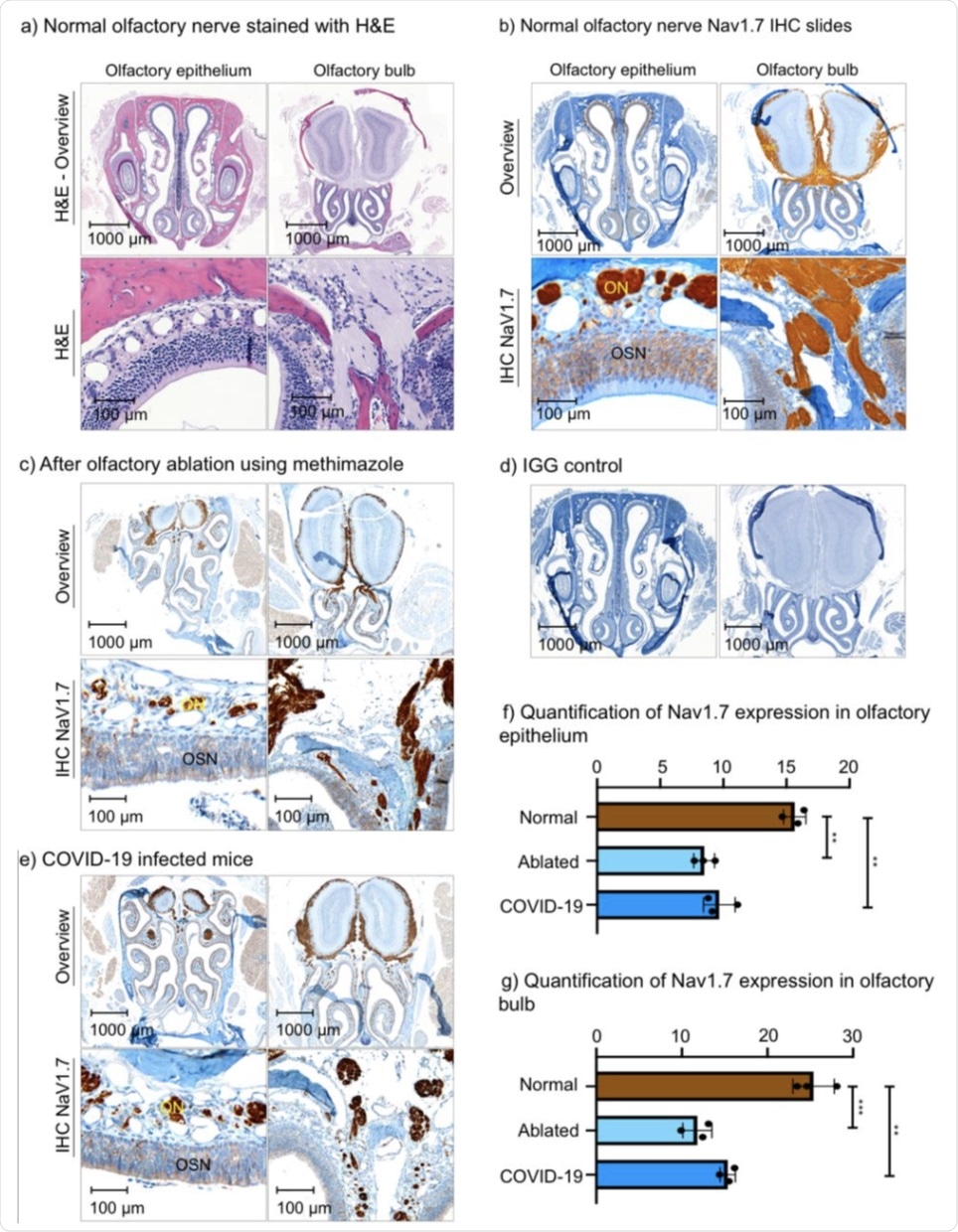 Histological slides of the olfactory bulb and olfactory epithelium of wild-type (WT) mice, mice with olfactory ablation, and a mouse infected with COVID-19. (a) H&E stain of WT mouse. (b) NaV1.7 IHC slide of WT mouse. (c) IHC slide of the mouse after olfactory ablation. (d) IGG control slide. (e) IHC slides of a mouse with COVID-19 infection. f) Quantification of NaV1.7 expression in the olfactory epithelium. (g) Quantification of NaV1.7 expression in olfactory bulb. ** P ≤ 0.01, *** P ≤ 0.001 ON – olfactory nerve bundles, OSN – olfactory sensory neurons, ONL – olfactory nerve layer.
Histological slides of the olfactory bulb and olfactory epithelium of wild-type (WT) mice, mice with olfactory ablation, and a mouse infected with COVID-19. (a) H&E stain of WT mouse. (b) NaV1.7 IHC slide of WT mouse. (c) IHC slide of the mouse after olfactory ablation. (d) IGG control slide. (e) IHC slides of a mouse with COVID-19 infection. f) Quantification of NaV1.7 expression in the olfactory epithelium. (g) Quantification of NaV1.7 expression in olfactory bulb. ** P ≤ 0.01, *** P ≤ 0.001 ON – olfactory nerve bundles, OSN – olfactory sensory neurons, ONL – olfactory nerve layer.
The scientists isolated and dissected olfactory bulb and epithelium regions from mice and stained them to reveal areas where Nav1.7 was expressed highly. To this end, they discovered that Nav1.7 is most highly expressed in olfactory nerves within the lamina propria, which is the connective tissue that lines the nose, followed by layers of primary OSNs. Expression decreases beyond the olfactory nerve layer.
This observation is supported by previous studies that revealed very similar findings. Olfactory ablation, which is defined as the damaging of nerves, and COVID-19 infection were shown to significantly decrease the expression of Nav1.1 from 15.7 in wild-type mice to 8.5% and 9.7% for ablated and COVID-19 infected mice, respectively. These same olfactory-ablated mice showed a significantly reduced ability to find buried food, with wild-type mice finding it under 30 seconds, compared with an average of 135 seconds for ablated mice.
Implications
The authors highlight the importance of a new diagnostic method for the detection of smell disorders that can identify the location of the issue, as well as show the significance of the damage and help guide the treatment. The imaging presented here showed very similar results to previous studies on Nav1.1. Following ablation, clear loss of Nav1.1 expression was observed, with the loss of expression correlated with the time mice spent attempting to find food in the buried food test.
Loss of smell through COVID-19 infection was more difficult to detect due to the nature of the infection that can cause anosmia through multiple mechanisms. However, several of those mechanisms can expose the OSN to environmental factors and cause inflammation in the area, leading to decreased Nav1.1 expression Therefore, the imaging agent can detect smell disorders caused by COVID-19.
As mass vaccination will hopefully bring the pandemic under control and ‘long COVID’ effects become more and more pronounced, technologies such as this could play a key part in helping to identify and treat symptoms. Moreover, this type of diagnostic approach could significantly reduce the burden on the health services, as well as improve the quality of life for many people.
The method discussed here is non-invasive, fast, and completely objective. Many commercially available endoscopes can already detect the wavelength emitted by the stain; therefore, it should be relatively easy for medical professionals to quickly and cheaply bring this system on board as a new diagnostic tool.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information
Content Source: https://www.news-medical.net/news/20211013/New-method-to-diagnose-anosmia.aspx